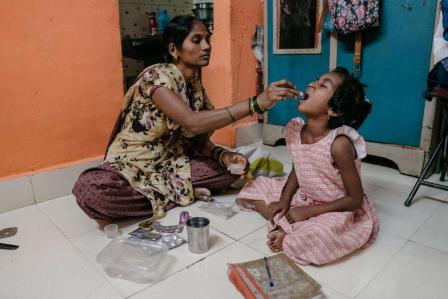MSF: Governments off track on providing tools to prevent TB, the second biggest infectious disease killer after COVID-19

Everybody Breathes is a package documenting how the lessons learnt from the initial phases of the ‘Bending The Curves’ initiative in South Africa are now being used by MSF in Eshowe to tackle TB. The package will document interventions the project is putting in place in collaboration with its partners to prevent the spread of TB, provide a link for patients to get tested, locate missing cases and ensuring patients remain on treatment with structures that fit into their lives. © Tadeu Andre/MSF
As the World Health Organization (WHO) convenes a ‘call to action’ tomorrow on the need to scale up access to preventive treatment for tuberculosis (TB), the international medical humanitarian organisation Médecins Sans Frontières/Doctors Without Borders (MSF) urged all governments to support and ramp up the implementation of TB preventive treatment (TPT), and demanded that pharmaceutical and diagnostics corporations make all drugs and tests needed to implement TPT accessible and affordable for those in need.
TB was only recently surpassed by COVID-19 as the world’s number one infectious disease killer. In 2019, TB claimed the lives of 1.4 million people and more than 10 million people fell ill. While treating people who are currently sick with TB is essential, another important measure to control and reverse the tide of the global TB burden is to promptly identify people at risk and prevent them from developing the disease through preventive treatment or TPT. TPT involves providing anti-TB drugs to family members and other close contacts of people sick with TB, to people with HIV or other conditions that weaken their immune system or to people otherwise at-risk, including people who are incarcerated. This preventive treatment will prevent people with latent TB (someone infected with TB, but not yet sick, contagious or showing symptoms) from developing full blown TB that could ultimately not only make them seriously ill, but also infect others. Scale-up of TPT is even more critical in the context of COVID-19. Urgent action is required to reverse the negative impact of COVID-19 on TB control.
In 2018, countries agreed during the United Nations High-Level Meeting on TB to meet the target of 30 million people started on TPT by the end of 2022. However, even though the effectiveness of TPT has been repeatedly demonstrated, progress is lagging behind. With only 18 months left, governments must urgently prioritise and scale up the implementation of preventive TB treatment to save more lives and alleviate suffering.
MSF aims to provide TPT as an integral part of the organisation’s comprehensive TB response. In Blantyre, Malawi, MSF supported a TB programme in the Chichiri prison. As people who are incarcerated – especially in high HIV- and TB-burden settings – are at a very high risk of being infected and developing TB, the programme screened for and treated people with TB, and provided TPT for people with latent TB infection and for people living with HIV. In 2019, the programme screened 1,500 people, and nearly two-thirds of those screened were started on TPT (666 HIV negative individuals and 324 people with HIV).
In Khayelitsha, South Africa, MSF has successfully provided TPT for children and adolescents who are contacts of people with drug-resistant TB (DR-TB). The COVID-19 pandemic required MSF to adapt its programme to start providing home-based care, which ultimately led to an increase in children and adolescents started on TPT through this community-based initiative.
TPT implementation is lagging in part due to a lack of access to the best tests and drugs. Most people only have access to the older TPT drug (isoniazid) that requires taking pills for 6-9 months. But a newer and shorter 1- to 3-month preventive treatment (rifapentine + isoniazid) exists, although it is more expensive (US$5 to $26.90 per month for the 3- and 1-month regimens respectively, versus $0.58 per month for the older single isoniazid-based
regimen). In addition, quality-assured rifapentine is only produced by two manufacturers (Sanofi and Macleods) because of limited demand, despite the fact that rifapentine has been off-patent for years, and it is not available in a child-friendly formulation. Further research into innovative alternatives that would make TPT easier, including long-acting injectable formulations of TPT drugs, would encourage greater scale-up.
Access to the diagnostic tools needed to screen widely for TB is also critical to ensure that healthcare workers provide TPT to the right people. More affordable and simpler point-of-care tests adapted to low-resource settings to identify people with latent TB are urgently needed to accelerate TPT implementation.